Of all the challenges facing the world today, two of the biggest are solving the problem of waste plastic and reducing fossil fuel usage.
One of the most important ways to burn less oil is to use more electric cars which have been charged up from renewable energy sources. To achieve this, a lot of electric car batteries will need to be made, which will in turn need a lot of raw material, such as lithium, cobalt, manganese, and nickel.
However, a team from the University of California, Riverside, believe that they have found a much cheaper way to make batteries by using nanomaterials constructed from waste plastic.
“Thirty percent of the global car fleet is expected to be electric by 2040, and the high cost of raw battery materials is a challenge,” said Mihri Ozkan, a professor of electrical engineering at the University of California, Riverside and the study’s co-author. “Using waste from landfill and upcycling plastic bottles could lower the total cost of batteries while making battery production sustainable on top of eliminating plastic pollution worldwide.”
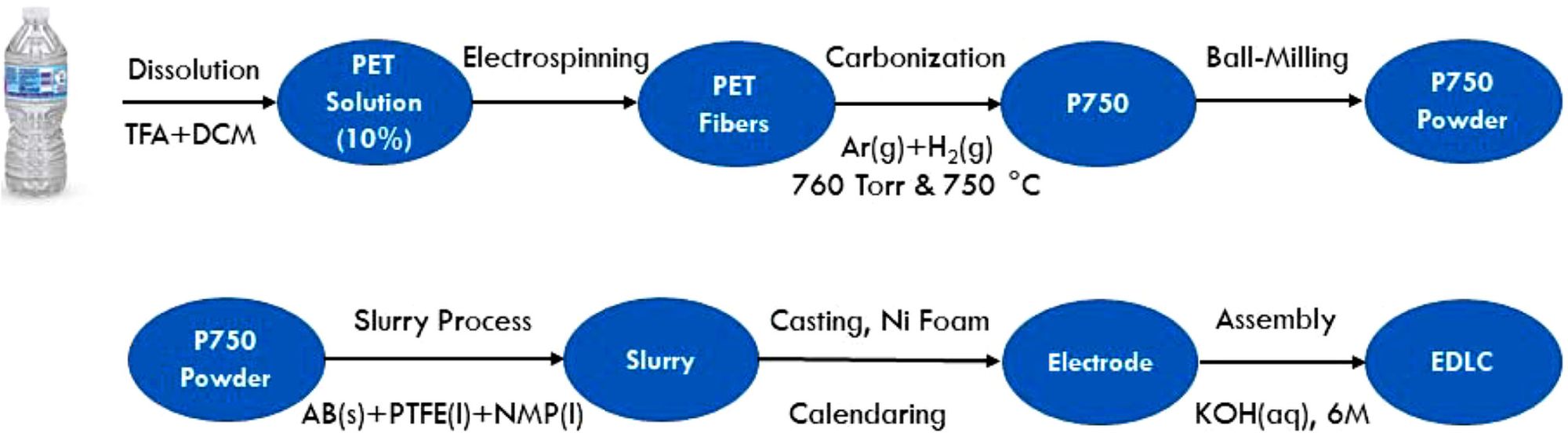
As the university’s press release explains, the research team, led by Ozkan and her husband and co-author Cengiz Ozkan, has developed a method for recycling polyethylene terephthalate (PET) plastic into a porous carbon nanostructure suitable for use in batteries. The team state that the new process is safe and sustainable, and can use everyday waste, such as plastic drinks bottles, shampoo containers, and vegetable oil bottles.

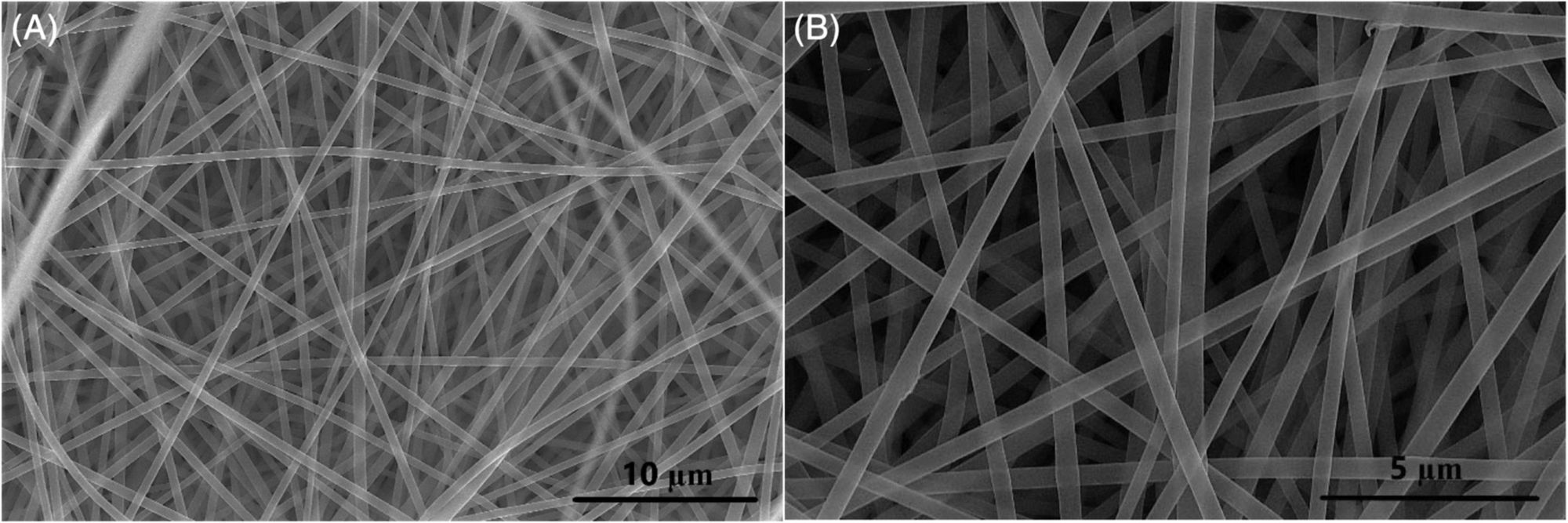
The process is relatively simple, considering the impact it could have on the battery and automobile industry, but begins by dissolving, “… pieces of PET plastic bottles in a solvent. Then, using a process called electrospinning, they fabricated microscopic fibers from the polymer and carbonized the plastic threads in a furnace. After mixing with a binder and a conductive agent, the material was then dried and assembled into an electric double-layer supercapacitor within a coin-cell type format.”
Analysis of this novel nanomaterial gave positive results. The press release stating that, “When tested in the supercapacitor, the material contained the characteristics of … a double-layer capacitor.” Specifically noting that, “Though they don’t store as much energy as lithium-ion batteries, these supercapacitors can charge much faster, making batteries based on plastic waste a good option for many applications.”
“At UCR, we have taken the first steps toward recycling plastic waste into a rechargeable energy storage device,” says the study’s first author Arash Mirjalili. “We believe that this work has environmental and economic advantages, and our approach can present opportunities for future research and development.”

The researchers have now published their work in the journal Energy Storage, which describes how, “The process simply consists of dissolution of the plastic, fiberization through electrospinning, and carbonization in a furnace.”
The study is also quick to highlight the environmental advantage of using plastic as a feedstock, stating that an estimated 6.3 billion metric tons (MT) of plastic has already been made, of which 9% has been recycled, 12% has been incinerated, and the remaining 79% has been dumped in landfill.
Finding a solution to this waste of resources is crucial.
As Cengiz Ozkan observes, “The upcycling of PET plastic waste for energy storage applications could be considered the holy grail for green manufacturing of electrode materials from sustainable waste sources. This demonstration of a new class of electrodes in the making of supercapacitors will be followed by a new generation of Li-ion batteries in the future, so stay tuned.”
Photo credit: UCR, EurekaAlert, & Wiley