The problem of brine is an expensive issue for the chemical industry.
A 2019 study conducted at Wageningen University estimated that global brine production is around 142 million cubic metres per day.
While 97% of the world’s water is naturally salty, brine cannot simply be dumped into the seas and oceans without environmental consequences.

“Brine underflows deplete dissolved oxygen in the receiving waters,” explains the Wageningen study’s lead author Edward Jones. “High salinity and reduced dissolved oxygen levels can have profound impacts on benthic organisms (such as worms, clams, crabs, lobsters and sponges that live in the seabed), which can translate into ecological effects throughout the food chain.”
Fortunately, industry uses up a lot of excess brine, with a 2018 analysis finding that, “In Europe, approximately 62% of salt consumption is attributed to chemical production (chlorine and caustic soda, sodium carbonate, sodium chlorate etc), followed by food processing (7.6%), de-icing (6.5%), water softening (3.7%) and many other uses.”

Unfortunately, industry produces a lot more than it requires, leading to brine treatment and disposal on a massive scale. An EU report stating that, “Process industries are a major source of brine discharge, with the [European] chemical industry alone producing 11.5 million tons of brine every year going along with high costs to manage.”
In fact, the chemical industry is the largest producers of brine, with EU data of brine generation in the Netherlands showing that, “… the chemical industry generates 52% of the chloride releases, followed by the waste and wastewater management sector (20%), the energy sector (13%), the production and processing of metals (11%) and the animal and vegetable sector from food and beverage (4%).”

“Industrial processes, in general, produce salty wastewater because the trend is to reuse water,” explains Prof. Qilin Li, co-director of the Nanotechnology Enabled Water Treatment Center (NEWT). “Many industries are trying to have ‘closed loop’ water systems. Each time you recover freshwater, the salt in it becomes more concentrated. Eventually the wastewater becomes hypersaline [when it is about ten times saltier than seawater] and you either have to desalinate it or pay to dispose of it.”
Knowing that the financial and ecological cost of so much salty water is a major headache for chemical companies everywhere, researchers from the universities of Yale, Arizona State, Rice, and Texas, including Prof. Li, have worked on a technology with nanomaterials to solve the problem of desalinating industrial-strength brine.
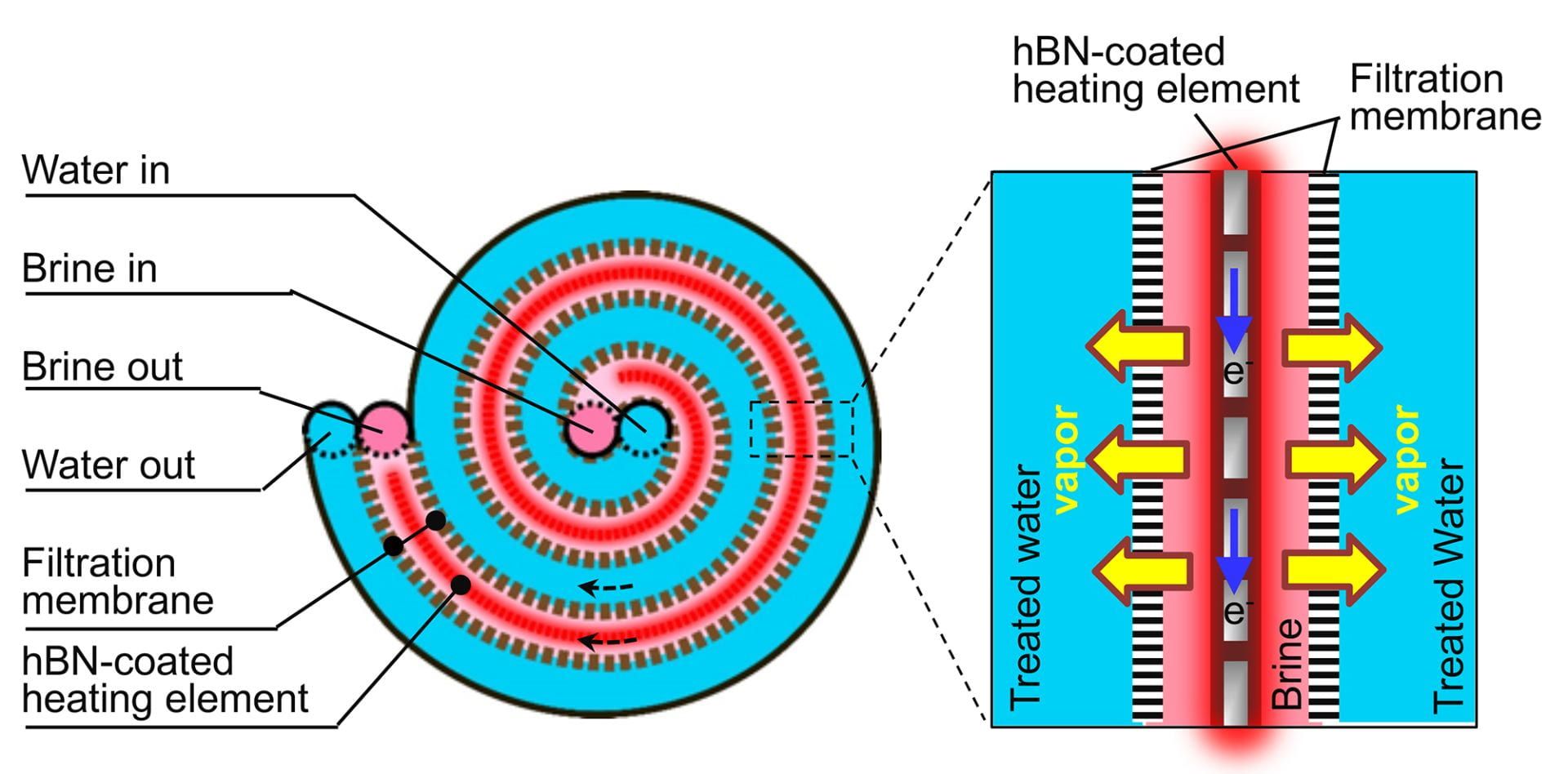
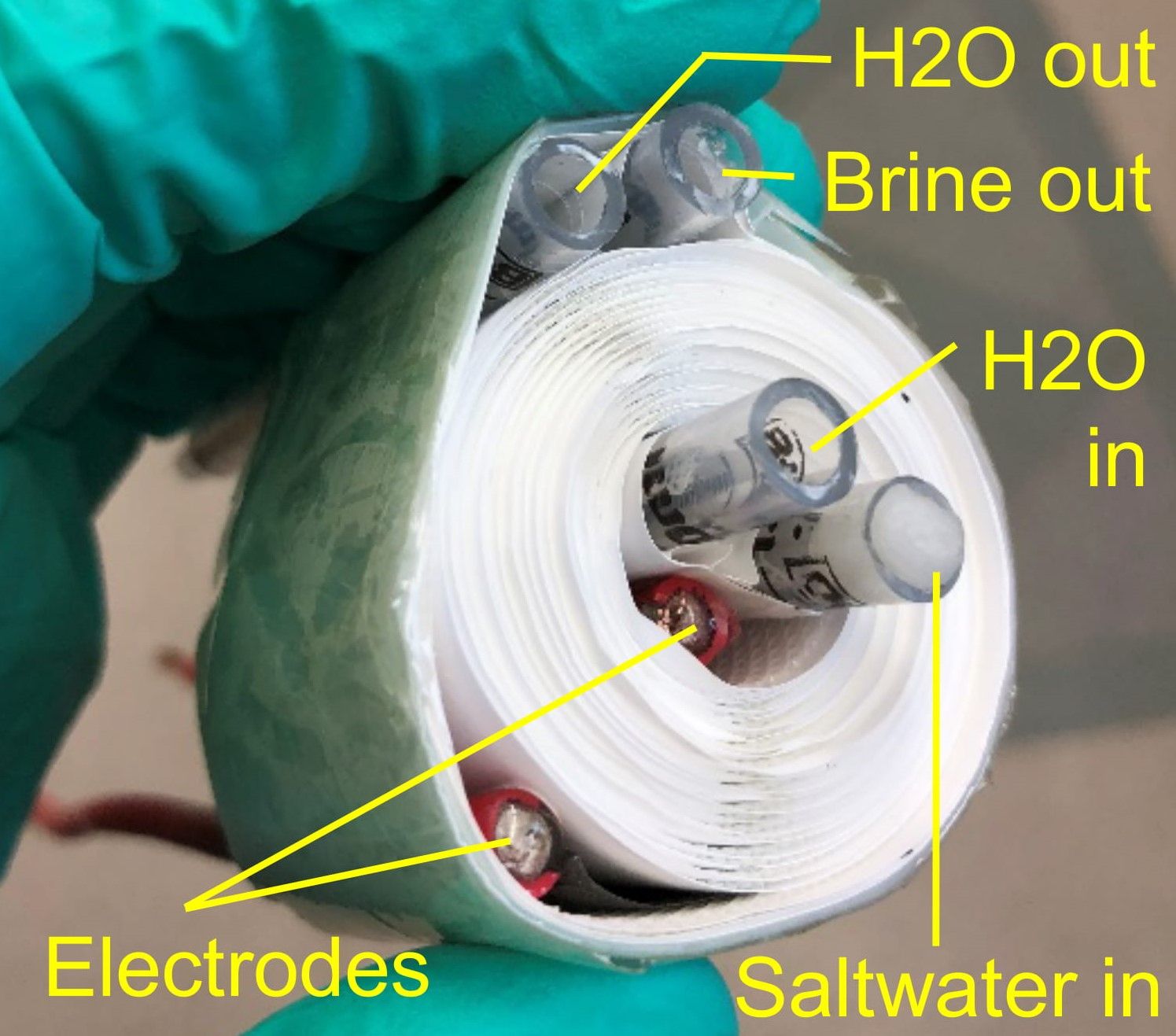
As the press release highlights, “[the] desalination technology for hypersaline brine features a central passage for heated brine that is sandwiched between two membranes. A stainless steel heating element produces fresh, salt-free water by driving water vapor through each membrane.”
Whereas most desalination processes require large amounts of power, this nanotechnology development has been created to work on solar power and a process called membrane distillation.
“When the brine is flowed across one side of a porous membrane, it is heated up at the membrane surface by a photothermal coating that absorbs sunlight and generates heat. When cold freshwater is flowed across the other side of the membrane, the difference in temperature creates a pressure gradient that drives water vapor through the membrane from the hot to the cold side, leaving salts and other nonvolatile contaminants behind.”
It is the difference in temperature that makes the process so simple and energy efficient, although the research team are still tweaking the process so that solar power alone can desalinate hypersaline water at an industrial rate.
“The energy intensity is limited with ambient solar energy,” said Li. “The energy input is only one kilowatt per meter square, and the production rate of water is slow for large-scale systems.”
The major problem with creating an industrial speed desalination plant is that salt is highly corrosive and becomes even more so when the water is heated. This can quickly destroy the heating elements, even if they are made out of non-metallic alternatives. Materials tested that are more resilient to salt, were found to have poor conductivity.
To solve this, the researchers turned to nanomaterials, which can be made strong enough to resist chemical corrosion whilst also maintaining sufficient conductivity to act as a coating on the heating elements.
“We were really looking for a material that would be highly electrically conductive and also support large current density without being corroded in this highly salty water,” notes Li.
The material they found which had all the required criteria was the 2D nanomaterial hexagonal boron nitride (hBN) which could be used as a coating to protect the heating element from the highly corrosive brine.
The challenge though, was getting the nanomaterial coating to form on a wire mesh. While boron nitride’s properties have long been useful as a thermally and electrically conductive, chemically robust material, it had previously only been synthesized on flat surfaces.
“This is the first time this beautiful hBN coating has been grown on an irregular, porous surface,” said Li. “It’s a challenge, because anywhere you have a defect in the hBN coating, you will start to have corrosion.”

This the researchers achieved through an adapted chemical vapor deposition (CVD) technique to “grow dozens of layers of hBN on a nontreated, commercially available stainless steel mesh”.
The result is a highly efficient and practical solution for desalinating industrial brine. As the press release states, “In tests, researchers powered the heating element with voltage at a household frequency of 50 hertz and power densities as high as 50 kilowatts per square meter. At maximum power, the system produced a flux of more than 42 kilograms of water per square meter of membrane per hour — more than 10 times greater than ambient solar membrane distillation technologies — at an energy efficiency much higher than existing membrane distillation technologies.”
Such is the success of the study, that the team are now looking to combine with industry to develop the project further.
“We’re ready to pursue some commercial applications,” says Li.
Given the pressing and increasingly expensive problem of industrial brine, there has already been plenty of interest in developing the process to an industrial scale.
This is understandable with demand for industrial chemicals increasing. Additionally, population growth and climate change are causing a surge in demand for limited freshwater resources when desalination plants are major brine producers.
The challenge of treating industrial quantities of brine will not go away overnight. Instead, practical solutions such as this may help to reduce the chemical industry’s water usage, waste output, and lower costs - all thanks to a nanomaterial coating.
Photo credit: Rice University, Patrick Hendry on Unsplash, pexels, pixabay, stocksnap, & pexels