Modern chemical products are all around us, from medicines to food flavourings, from plastics to coatings. And most of these chemical products are synthetically derived molecules, devised in a lab after years of experimentation. To get to these products, chemists often turn to catalysts to create and control chemical reactions.
Catalysts can be thought of as additives which make something new or unexpected happen. The standard process used for conducting the large number of experiments to find a novel catalyst is called ‘screening’. It is the established practice used, particularly in pharmaceutical research, when searching for active chemical ingredients.
While running a screening program to develop catalysts requires a good understanding of chemistry, the breakthrough is often made by chance. As such, it often takes many months of testing before a new, valuable catalyst is found.
Given the high cost of chemical research and the significant value that new chemical products have, chemical companies waste millions of dollars simply hoping for a profitable accident to happen. To avoid waiting for these lucky mistakes, a team of researchers from the University of Munster in Germany have developed a systematic strategy for generating the ‘random hits’ which create successful unexpected reactions.
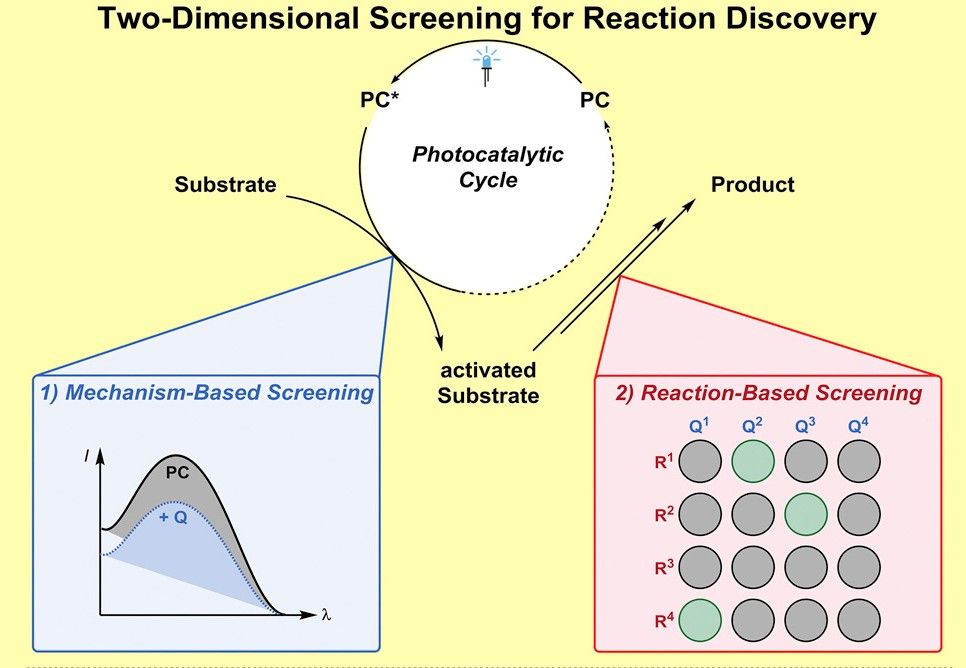
This new method of screening involves two separate steps which looks at a variety of different elements in a chemical reaction, and which only in combination are designed to find new synthetically relevant reactions.
The online journal Phys.org describes the beginning of the process as follows; “In the first step, chemists examine whether a potential substrate actually interacts at all with the catalyst. For this purpose, in the case of photo-catalysts, the phenomenon of emission quenching is used. If a substrate reduces the emission of the catalyst, an interaction between catalyst and substrate is likely. By systematically screening a large number of randomly selected compounds, new molecules can be identified whose interaction with catalysts was previously unknown.”
However, as the interaction between the substrate and the catalyst does not by itself create a reaction, a second screening process is added. This step involves, “examining whether a reaction does actually take place when a reaction partner and the catalyst are present”.
At this point, by merging the results from the two screening steps both partners of a new reaction can be identified, leading to the development of a new chemical product. As the study, now published in the journal Chem, states, The discovery of novel (catalytic) transformations and mechanisms is commonly based on rational design. However, many discoveries have resulted directly from experimental serendipity [luck]. Building on this, we report a two-dimensional screening protocol, combining ‘mechanism-based’ and ‘reaction-based’ screening and its application to the field of visible light photocatalysis.” Adding that, “Such screening approaches can provide complementary access toward novel and more efficient catalytic protocols.”
As Prof. Frank Glorius from the Institute of Organic Chemistry at Münster University, notes, “This two-dimensional strategy enables us not only to find new catalyst-substrate interactions, but also to actually discover new reactions - including some we hadn't previously expected.”
Already this new approach to chemical research is yielding results, as the study includes three previously unknown chemical reactions which were identified by the two-step screening. As the study’s lead author, Felix Strieth-Kalthoff, explains, “As formulated on paper, I would not have considered this reaction to be possible, because, from an energetic point of view, the key step in this reaction shouldn't actually be possible.”
Thanks to the new strategy, says Dirk Guldi from the University of Erlangen, who is considered to be one of the world's leading experts on photochemical processes, “We're now able to provide much better explanations for the underlying molecular processes in the triplet-triplet energy transfer - the key activation step.” Before adding that, “This greater understanding will allow the development of new processes and catalysts.”
But the team are not stopping with just a strategy. Instead, they are continuing their research and applying their theory towards discovering even more new catalysts. By combining the latest computer technology and other research techniques with their two-step screening approach, the team have found ther new theories on screening, as well as new classes of reactions.
Such discoveries have certainly got the Munster team excited and is sure to draw attention from the rest of the scientific community as well as industrial chemical researchers. As Glorius states, “We are convinced that this strategy can be used in other areas of catalysis - and beyond.”
Certainly, the fact that the study was able to note three new catalytical reactions goes some way to prove what a game-changer ‘two-step screening’ could be for the entire chemical industry. As Glorius concludes, “I believe that the discovery of new types of reactions resulting from data-based strategies, such as these screening methods, will make a decisive difference to the development of synthetic chemistry.”
Photo credit: UniversityofMunster, Matthey, & UNSW