With the ongoing growth of electrical devices has come the ongoing demand for better batteries. This was unofficially acknowledged when the 2019 Nobel Prize for Chemistry was awarded to John Goodenough, Stanley Whittingham, and Akira Yoshino for ‘the development of lithium ion batteries’.
While the first commercial lithium-ion battery was produced by Sony as recently as 1991, today they are found in countless devices such as mobile phones, tablets, and laptops. Their diverse usage is due their high energy density and low maintenance. They are also relatively safe, (despite a handful of mass recalls), such that in 2015 660 million li-ion cells were produced. Meanwhile the 2020 market for li-ion batteries in handheld devices is predicted to be worth $7 billion, with a further $10.2 billion in electric cars.
However, lithium-ion batteries are far from perfect, especially as they can suffer a short life span, often with a notable drop in performance when as little as one year old, even without being used.
Improving lithium-ion batteries has therefore often focused on the fact that they are difficult to recycle. The other major drawback is that they are fragile, and so must be handled with some care and must avoid being dropped or hit.

These problems may now have been solved as a team from the University of Illinois have developed a solid polymer-based electrolyte for use in lithium-ion batteries. Not only will this allow for batteries that can self-heal, as well as be recycled without the need for high temperatures and harsh chemicals, but the new design will lessen the chance of fires that have been a constant risk in liquid electrolytes.
The design fault in current lithium-ion battery technology is outlined in the University of Illinois press release, which explains that, “As lithium-ion batteries go through multiple cycles of charge and discharge, they develop tiny, branchlike structures of solid lithium called dendrites …. These structures reduce battery life, cause hotspots and electrical shorts, and sometimes grow large enough to puncture the internal parts of the battery, causing explosive chemical reactions between the electrodes and electrolyte liquids.”
To avoid these issues, researchers have long sought raw materials to make solid electrolytes, experimenting with different ceramics and polymers. However, most of these materials were found to be too brittle and rigid, and resulted in “poor electrolyte-to-electrode contact and reduced conductivity”.
As Brian Jing, a materials science and engineering graduate student and one of the study’s co-authors, highlights, “Solid ion-conducting polymers are one option for developing nonliquid electrolytes, but the high-temperature conditions inside a battery can melt most polymers, again resulting in dendrites and failure.”
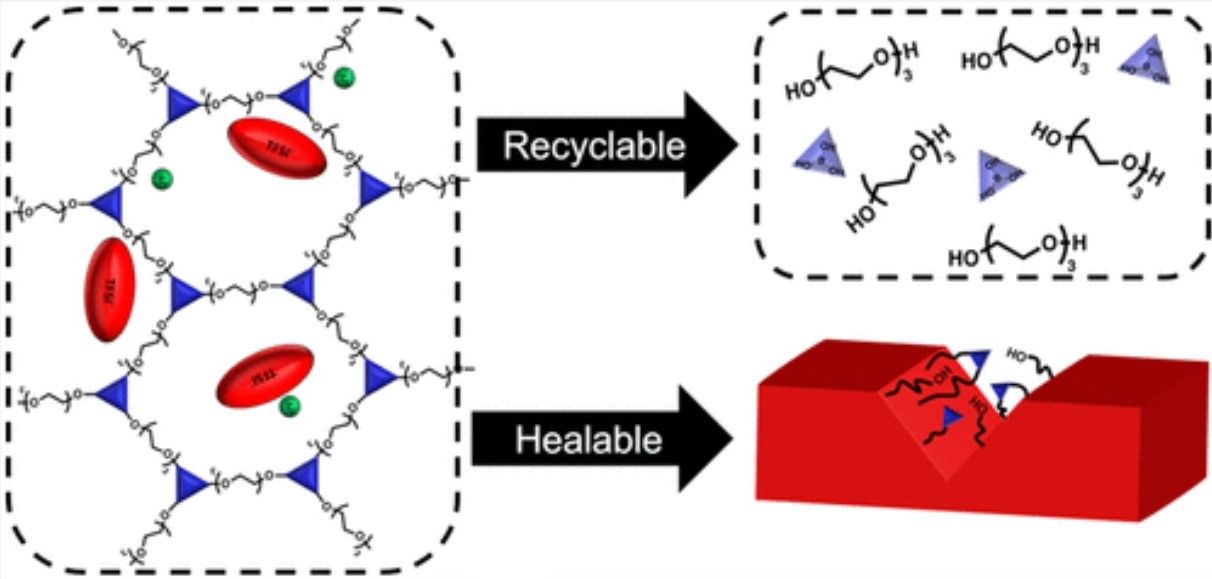
Instead, attempts were made to create solid electrolytes using polymer strands that were cross-linked to make a rubbery lithium conductor. While this proved successful in limiting the growth of dendrites, the materials were complex to make and could neither heal themselves nor be easily recycled.
In contrast to this approach the Illinois-based team developed a network polymer electrolyte in which the cross-link point can undergo exchange reactions and swap polymer strands. As the online journal Science Daily explains, “In contrast to linear polymers, these networks actually get stiffer upon heating, which can potentially minimize the dendrite problem. Additionally, they can be easily broken down and resolidified into a networked structure after damage, making them recyclable, and they restore conductivity after being damaged because they are self-healing.”
The result is a very special battery design. “This new network polymer also shows the remarkable property that both conductivity and stiffness increase with heating, which is not seen in conventional polymer electrolytes,” says Jing.

“Most polymers require strong acids and high temperatures to break down,” adds Christopher Evans, a professor of engineering and materials science and the study’s lead author. “Our material dissolves in water at room temperature, making it a very energy-efficient and environmentally friendly process.”
The study has now been published in the Journal of the American Chemical Society, where they describe the creation of, “poly(ethylene oxide)-based networks with varying amounts of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) to understand the impact of a salt on the ion transport and network dynamics.” Significantly discovering that, “these networks can efficiently dissolve back to pure monomers and heal to recover their conductivity after damage, showing the potential of dynamic networks as sustainable solid electrolytes.”
Early results have found that the new material has great promise for use as an effective battery electrolyte, with good conductivity, a safer design, and a better chance at low-cost recycling. Although the team are quick to acknowledge that further work is required to develop the material for use in a modern battery.
“I think this work presents an interesting platform for others to test,” observes Evans. “We used a very specific chemistry and a very specific dynamic bond in our polymer, but we think this platform can be reconfigured to be used with many other chemistries to tweak the conductivity and mechanical properties.”
The development of safer, self-healing, and recyclable lithium-ion batteries could mean so much more than better mobile phones, as they could form the backbone of future transport. For example, at present, lithium-ion batteries have power conversion rates as high as 90%, compared to 34% for petrol driven cars.
With car fires causing bad publicity for both electric car and mobile phone manufacturers, alongside questions over environmentally friendly disposal, the development of self-healing, recyclable lithium-ion batteries could be the breakthrough the automobile and electronics industry has been waiting for.
You may also be interested in reading; The Next Big Thing for Raw Material Sourcing; Lithium Recycling.
Photo credit: Journal of the American Chemical Society, University of Illinois, Vox, Indianexpress, & Smartphonebattery, Pexels