The world is at war against a deadly virus. An enemy whose many waves of attack will take may lives and alter the economy, politics, and society at large for years to come.
To minimise our losses and to quickly return life to normal requires a better understanding of the virus. Only then can we begin to stop it.
As the great war strategist Sun Tsu said, “If you know the enemy and know yourself, your victory will not stand in doubt.”
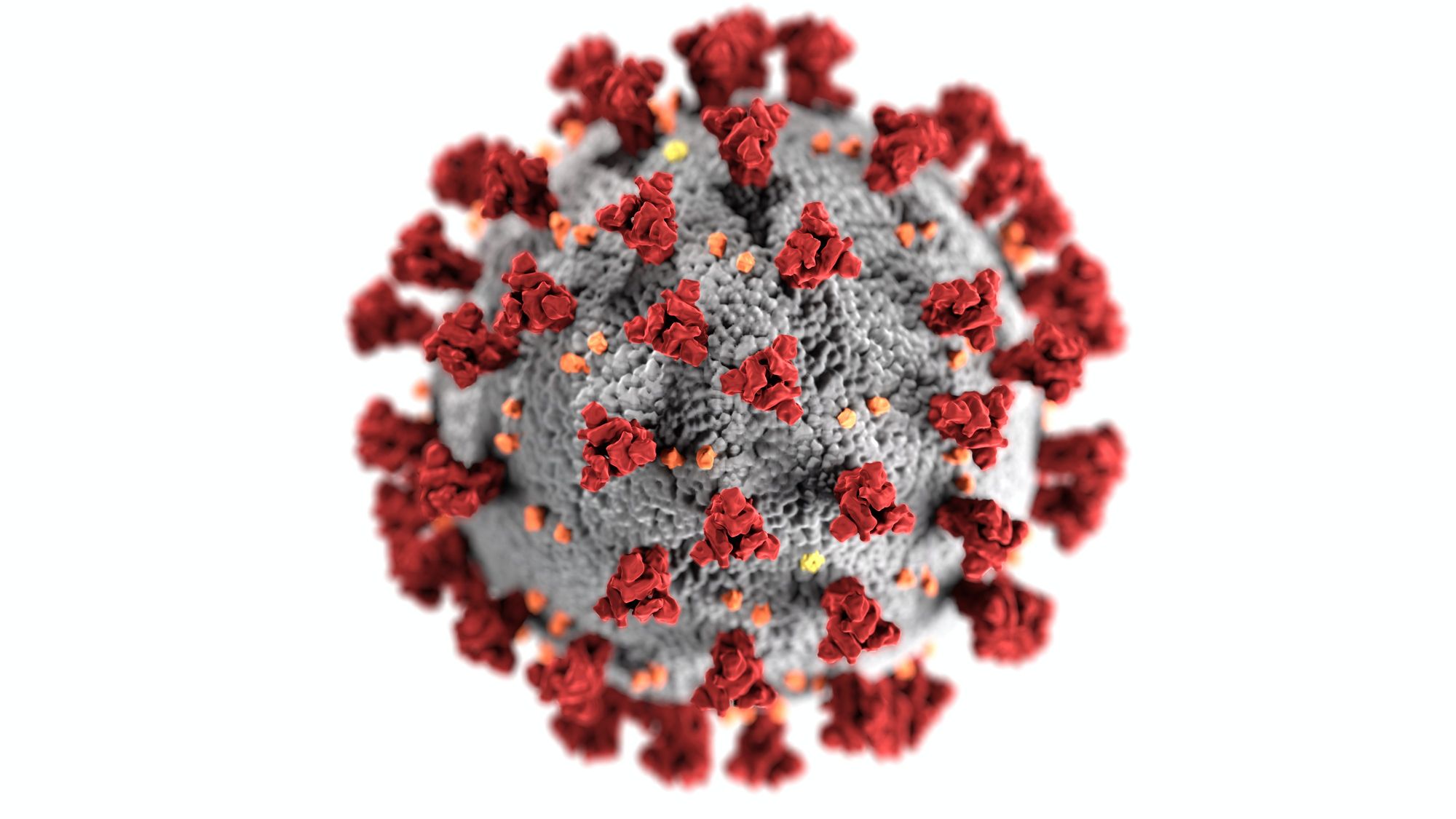
Dr. Swapan Kumar Ghosh, a director at the Nova Surface-Care Centre and a polymer scientist with over 25 years of research experience in academia and industrial R&D, describes the virus as, “… an enveloped, positive-sense, single-stranded RNA virus. Coronaviruses are so named because of their characteristic solar corona (crown-like) appearance when observed under an electron microscope.” Noting that, “This appearance is produced by the peplomers of the surface (or spike; designated S) glycoprotein radiating from the virus lipidenvelope. The SARS-CoV virion is spherical with an average diameter of 78 nm.”
Like many viruses, it is spread from person to person via the water droplets that carry in the air from sneezes, coughs, and talking. If inhaled, or enters the body through the eyes, the virus may infect another victim.
Besides its high rate of fatality, what makes coronavirus exceptional is that it can survive on surfaces for many days. Even if the surface looks clean, an unsuspecting victim using a handrail, lift button, or even money that has come into contact with the virus can transfer it from the object into their body, and so become sick.
The first lines of defence are simple; minimise contact with others, wash your hands frequently, stay 2m apart from others where possible, and wear a mask.
Additional layers of protection involve the use of disinfectant gels and sprays, but all these methods offer only a temporary defence.
As the industry journal Coatings World explains, “Though the coronaviruses are relatively easy to destroy, using simple disinfectants like ethanol (62-71%), hydrogen peroxide (0.5%) or sodium hypochlorite (0.1%) by breaking the delicate envelope that surrounds the tiny microbe … It’s practically impossible to sanitize the surfaces all the time and it doesn’t guarantee that the surface won’t get contaminated again.”
Consequently, research is now focused on developing surfaces or surface coatings that can repel, destroy, or neutralize pathogens over a longer period.

Many studies in this field are based on how the spike glycoprotein in the virus enables it to bind with proteins on the surface of human epithelial cells in the lungs and respiratory tract. Finding a way to inhibit this connection could be key to stopping infection spread.
Surface coatings that can interfere or inhibit the spike glycoprotein can prevent the virus from landing on a surface, or destroy the virus, or stop its ability to connect with human cells.
At the heart of studies into ‘non-stick’ and ‘virus-killer’ surfaces are metallic nanocoatings, which have already been proven effective against bacteria, fungi, algae pathogens, as well as some viruses.
As Coatings Word reports, “AgNPs [silver nanoparticles] have been reported to inhibit the replication of virus nucleotides, the main mechanism of its being virulent. It binds to electron donor groups such as Sulfur, Oxygen, and Nitrogen commonly found in enzymes within the microbe. This causes the enzymes to be denatured thus effectively incapacitating the energy source of the cell and the microbe will quickly die.”
Previous works on the effects of nanoparticles on SARS, HIV, Hepatitis, and other viruses are numerous.
For example:
· A study published in 2019 in the Journal of Biomedical Science, reported how Zinc Oxide nanoparticles (ZnONPs) were effective in inhibiting H1N1 virus.
· The National Library of Medicine carries a study showing the ‘Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus’.
· As early as 2005, silver nanoparticles were developed that could interact with HIV-1 in the journal Nanobiotechnology.
· A 2011 study titled, ‘Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus’ was published in the journal Applied and Environmental Microbiology.
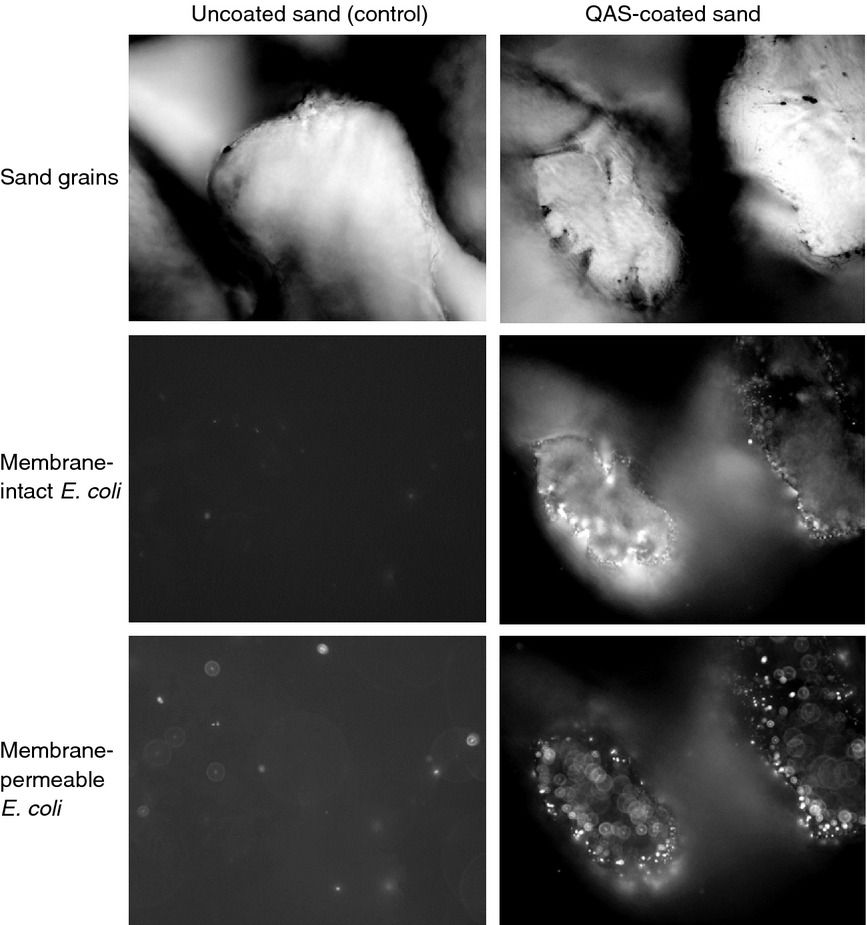
· Other works have listed the ability of gold and silica nanoparticles (Au-SiO2NPs), as well as ‘some Quaternary ammonium cations commonly called QUATs’ were reported to have a very promising ability to inactivate viruses in the journal of the Society for Applied Microbiology.
Alongside their effectiveness against viruses, nanocoatings have a significant advantage in the versatile range of surfaces they can be used on. Plastics used for switches and buttons, wood used in furniture and flooring, metals in lifts, door handles, and railings, and even fabrics used on masks, bed sheets, curtains, and clothes, can all potentially be protected by simple metallic nanocoatings.
However, until the nanotechnology is developed that specifically blocks the coronavirus’s ability to spread, we must remain vigilant in spraying these materials with regular disinfectants.
We are at war. We are working on the technology to win the war. Until that arrives, win the battles in your world with disinfectant sprays and gels.
Photo credit: Sfamjournals, CDC from Pexels, Polina Tankilevitch from Pexels, & Anna Shvets from Pexels