For decades, chemists have been looking for a way to convert carbon dioxide into chemicals. Not only would this lessen the chemical industry’s use of fossil fuels, but it would also lower the amount of CO2 the industry emits into the atmosphere each year. While there has been some success in converting carbon dioxide into basic chemicals, the range available at present is very limited.
However, a recently published study by a combined US/Chinese team have now found a way to make far more complex chemical structures from CO2, in a process that could lead to industrial scale carbon capture and utilization.
“In the field of carbon dioxide conversion, we were stuck with only four major products we can make using this technology,” explains Feng Jiao, an associate professor of chemical and biomolecular engineering at the University of Delaware and the study’s lead author. “Ethylene, ethanol, propanol, and, as we reported just a couple months ago in Nature Catalysis, acetate. Now, starting with carbon dioxide as a carbon source, we can expand to a variety of products.”
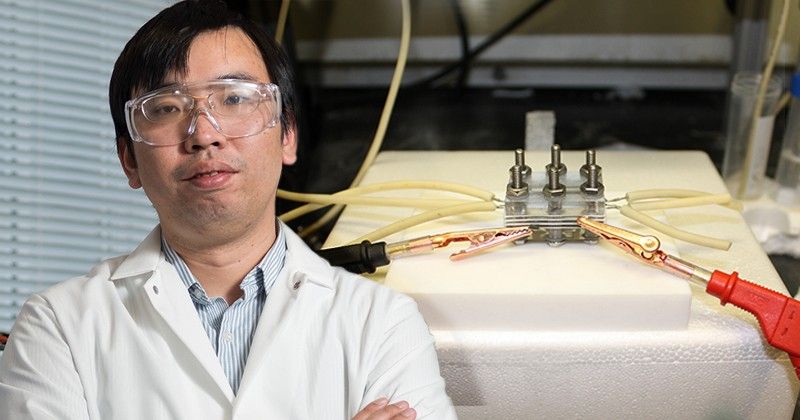
The basis of the discovery was electrochemistry, which uses electricity to produce chemical change, a field where Jiao is a leading expert. His earlier experiments involved a specialized silver catalyst, which was able to convert carbon dioxide to carbon monoxide. However, the ability to further upgrade carbon monoxide into more complex, multi-carbon products that could be used for producing fuel, pharmaceuticals, or specialty chemicals always remained a goal.
To achieve this, the team formed carbon-nitrogen bonds in an electrochemical carbon monoxide reduction reaction. This led to the production of high-value chemicals called amides, which can form the building blocks used in a variety of chemical sectors. As Jiao notes, the new route, “… provides a unique way to build large molecules which contains nitrogen from simple carbon and nitrogen species.”

In fact, it was the use of nitrogen that was key, as the University of Delaware (UD) press release explains, “Nitrogen is the secret ingredient to unlock the potential of the system. The team used an electrochemical flow reactor that is typically fed with carbon dioxide or carbon monoxide, but this time they put in both carbon monoxide and ammonia, a compound that contains nitrogen. The nitrogen source interacts with the copper catalyst at the electrode-electrolyte interface, leading to the formation of carbon-nitrogen (CN) bonds. This process allowed the team to synthesize chemicals that had never before been made in this way, including amides, which can be used in pharmaceutical synthesis.”
The team from UD, the California Institute of Technology, Nanjing University (China), and Soochow University (China) have now published their results in the journal Nature Chemistry, where they state, “… that C–N bonds can be formed through co-electrolysis of CO and NH3 [ammonia] with acetamide selectivity of nearly 40% at industrially relevant reaction rates.”
This high reaction rate makes the team is optimistic that the process can be scaled up. They are also optimistic that the process can be developed to construct other chemical products and compounds from CO2. This is based on analysis by the Goddard lab at the Joint Center for Artificial Photosynthesis at Caltech, which, “… found that the new carbon-nitrogen bond coupling was an off-shoot of the mechanism that had been determined for the production of ethylene and ethanol, suggesting that Jiao might be able couple bonds other than CN.”
“Through a close collaboration with Prof. Goddard, we learned quite a lot in terms of how this carbon-nitrogen bond formed on the surface of the catalyst,” says Jiao. “This gave us important insights on how we can design even better catalysts to facilitate some of these kinds of chemical reactions.”
Such a breakthrough is a big deal in the battle against climate change. The International Energy Agency estimates that the chemical and petrochemical industry released 1.25 Gt of CO2 in 2017, a 2% increase on the previous year. And while other industries (light-automobile, construction, power) are reducing their share of oil consumption, demand in the chemical industry remains strong, with BP’s 2018 Energy Outlook predicting that, “Oil consumed by … chemicals will continue to grow, from 16% of oil demand in 2020 to 20% by 2040.”
In global terms, the importance of developing industrial scale carbon capture and utilization technology is not missed by Jiao. “This has significant impact down the road to partially address carbon dioxide emission issues,” he says. “Now we can actually utilize it as a carbon feedstock to produce high-value chemicals.”
Photo credit: Newswise, Moneycontrol, Researchgate, & NCBI