A team of chemists have found a simple, cost effective way to stop the ‘coffee ring effect’ that causes imperfections when solvents evaporate from a solution containing non-volatile particles. The novel approach has already been successfully tested on numerous substrates, including polymers, graphene, and graphite, and is predicted to be a further tool in improving coatings and dyes.
Most people are aware of the ‘coffee ring effect’ from the circular stain tea or coffee leaves when it runs down the side of a cup. The fluid collects at the drinking vessel’s base, and when the moisture evaporates the sediment spreads to the edges where it dries out on the surface.
This phenomenon is in effect on most liquids and is a cause of concern in the printing, dyeing, and coating industry. For this reason, the way that sediments in liquids dry out has been thoroughly analysed.
The ‘coffee ring effect’ operates in the way that a drop of liquid is often pinned to a surface. As the liquid evaporates, the surface tension keeps the edges in place forcing the droplet to flatten out as its volume decreases. This pushes the liquid and any particles suspended in it to the edges. When the last water leaves, the particles are left dried out in an unsightly ring.
While a dirty circle of tea or coffee can easily be cleaned up, this is not possible with permanent dyes, inks, and paints. Consequently, the chemical industry has spent a lot of time and money trying to solve the problem.
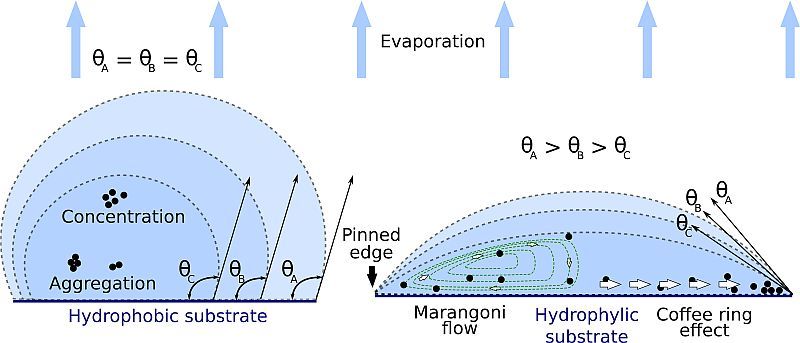
Attempted solutions include adding polymers, sol-gel inducers, surfactants, co-solvents, and proteins. Some studies even examined the effect of different shaped particles in coatings or experimented with electrical fields and external optics to control ring formation. While all of these answers had different degrees of success, none could truly solve the problem.
Now a team of coating specialists from East China University of Science and Technology and Shanghai University believe they have found the answer with a technique that employs trace amounts of various salts.
As the scientific journal Physics World reports, “When the researchers tested their technique on a solution containing dyes placed on graphene, polymers and other substrates that contain chemical structures known as aromatic rings, they found that the colour of the dye remained even [uniform] across the substrates. Molecular dynamics simulations revealed that strong interactions (known as cation-π interactions) between the hydrated cations in the solution and the aromatic rings in the substrates work to inhibit the coffee-ring effect, since they promote a more uniform adsorption of suspended matter onto the substrates.”
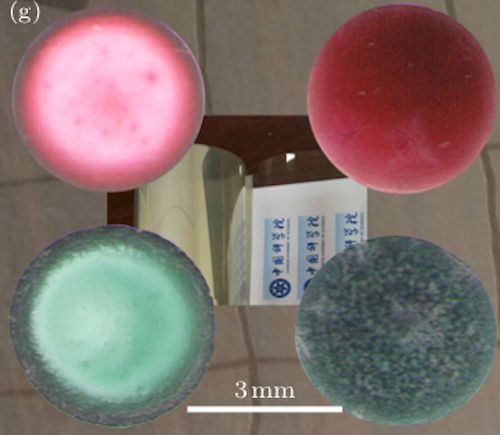
Led by the researchers Haiping Fang and Guosheng Shi, the team created solutions with different concentrations of sodium chloride (NaCl) by mixing the salt into aqueous suspensions of polystyrene microspheres. They also created a non-saline control.
These solutions were then placed in droplet form onto a graphene substrate. As the drops slowly evaporated in a temperature of 10° C they studied the rings that were left with optical microscopes and greyscale analysis.
What the team found was that, “In suspensions without NaCl, they observed ring-like patterns with a dark rim and a light grey centre on the graphene. For the salty suspensions, however, the rings were absent, and the image contrast between the rim and the centre of the pattern gradually diminished for mixtures with increasing NaCl concentrations. At a concentration of 8.0 mM NaCl, the pattern appeared completely uniform.”
The team have now published their results in the journal Chinese Physics Letters, where they state that, “By only adding trace amounts of salt into the suspensions, here we experimentally achieve the facile and highly efficient control of the coffee ring effect of suspended matter on substrates of graphene, natural graphite, and polyethylene terephthalate surfaces.”
The team have also conducted further testing with different salts, such as potassium chloride (KCl), calcium chloride (CaCl2), lithium chloride (LiCl), and magnesium chloride (MgCl2), again with varying concentrations, and were able to control the ring effect in a similar way.
They also had positive results when testing the process on other aromatic-ring substrates, such as natural graphite and importantly, the common thermoplastic resin polyethylene terephthalate (PET). Although, they were unsuccessful when experimenting on glass substrates.

While the problem of rings from drying liquids may seem a small matter to the man on the street. It has long been the bane of many industries. Effective drying of solutions is a chemical process that influences both coating and dyeing companies, as well as the printing business. It can also be a costly obstacle in complex assembly lines for micro and miniature products and would doubtless have asked many questions in the growing nanotechnology industry. The fabrication of nanoscale surfaces and the uniform deposition of chemicals being at the core of next-generation computing and electronics.
But in the end, it turns out the challenge of the coffee ring effect has a very simple, yet slightly salty solution.
Photo credit: PhysicsWorld, Behance, Advancedsciencenews, Goingconcern, Pinterest & Wiley Pixabay