Crude oil extraction can be a sticky, messy business.
While we typically imagine oil being cleanly pumped up from an underground reservoir, it is more likely that the oil is mixed in among gravel or squeezed into the rock itself. This is a major challenge for oil companies, decreasing efficiency in the oil extraction process when trying to draw out the last remnants of crude oil from a source.
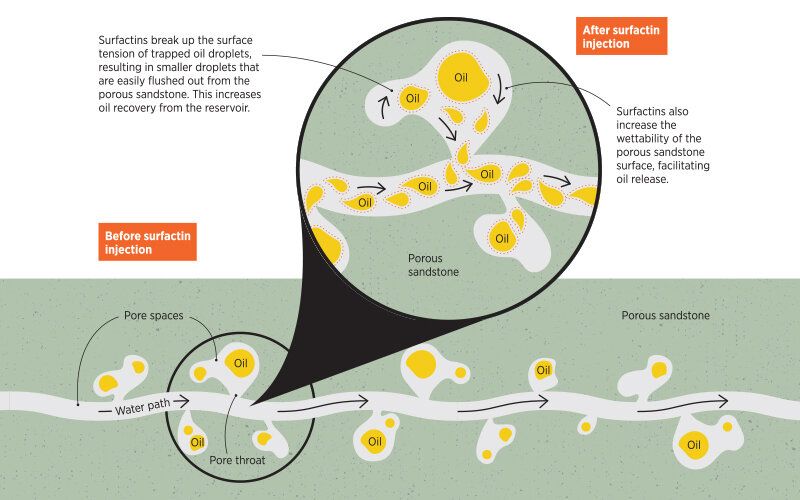
As Nanji Hadia, a Research Scientist at A*STAR's Institute of Chemical and Engineering Sciences (ICES), explains, “In most oil reservoirs, a large amount of oil remains unrecoverable because it is stuck in the micron-scale pore throats of reservoir rocks. Capillary forces are a key factor which holds back the oil in these tiny pore throats.”
To avoid this waste, the chemical industry has devised a range of techniques, known as enhanced oil recovery, to collect as much of the oil as possible.
“One type of enhanced oil recovery involves the use of surfactants to overcome the capillary forces holding the oil in place and allow oil and water to mix into emulsions which can then flow more easily through the pores,” says Hadia.
The problem with these techniques is that they use synthetic surfactants which contain non-biodegradable sulfates and sulfonates. While for the most part they are extracted with the oil for treating, disposal and/or recycling, some residues remain trapped underground. From here they can contaminate the water table or pose environmental concerns.
“Surfactins are known for their ability to drastically reduce the interfacial tension between oil and water,” notes Hadia. But this comes at a price. “Because of increasingly stringent environmental restrictions,” he adds, “it is a priority for the petroleum exploration and production industry to move towards green surfactants.”
With this in mind, Hadia joined forces with his colleague Christoph Ottenheim, with the goal of making a bio-produced enhanced oil recovery product; a surfactant-like molecule produced by bacteria.
Earlier work in this field has produced two types of biosurfactants that are commonly used by oil companies: lipopeptides, which are fat molecules with a short chain of amino acids attached to them, and glycolipids, which are fat molecules with a sugar chain attached.
Understanding this, Hadia and Ottenheim decided to focus on a lipopeptide called surfactin, which is produced by the Bacillus family of bacteria. While this chemical product is already well understood, the challenge had always been how to produce enough surfactins at a reasonable price. It was a problem that had perplexed the oil industry for a long time.
As the scientific journal Phys.org, reports, “Currently, surfactin is obtained by fermentation, whereby Bacillus are provided with a specific mix of nutrients and kept under precise pH, temperature and aeration conditions that favor surfactin synthesis.”
“In our case,” says Ottenheim, “we used Bacillus subtilis 22.2 strains and allowed them to ferment a nutrient broth overnight at 30°C.”
This fermentation process yielded a mixture of surfactins, albeit less than 1 gram of product per litre of broth.
The key to Ottenheim and Hadia’s work was in the effectiveness of the surfactins they produced.
As the report states, “Evaluating the effect of their surfactin mixture on two stock tank crude oils, the researchers found that a low surfactin concentration of 0.025% was able to reduce the interfacial tension of both oils by a hundred to a thousand-fold, thereby facilitating the formation of oil-in-water emulsions. This in turn allows oil to flow easily from pore throats.”
In further experiments designed to mimic real-world conditions, the surfactin mixture was used in ‘coreflooding tests’ where crude oil was trapped inside a natural, porous material called Berea sandstone. The results showed that the biosurfactin was highly efficient at extracting oil residues from inside the rock.

“We were able to recover 1.5-5% more oil by injecting 0.1% surfactin solution into the rock samples [as compared to when surfactin was not used],” Hadia said. A result that will improve efficiency in the oil extraction industry, as well as lowering the environmental impact of oil extraction. But beyond those gains, the discovery also highlights the advancing role of research into microfluidics; a branch of chemistry that analyses the way that liquids function at the very small scale.
“In the case of enhanced oil recovery studies,” says Hadia, “microfluidics helps researchers understand the displacement of one fluid by another…”
The research also further underlines the power of the surfactant industry.
While it can often be viewed by the public as a chemical sector that manufacturers dishwashing liquid, surfactants have a far wider reach than that.

As Dr Freda C H Lim, a computational chemist at the Institute of High Performance Computing (IHPC), noted in an interview with the industry journal World of Chemicals, “Surfactants play an important role in many areas of our lives, from cleaning to wetting and dispersing, from emulsifying to foaming and antifoaming. With such a myriad of application functions, prominent industry sectors that will benefit from research on surfactants are the consumer goods and personal care sector as well as the oil & gas, mining sector.”
As a case in point, this study shows that biosurfactants can even boost oil production.