In recent weeks, studies published by researchers from the Universities of Illinois, Davis-California, and Wake Forest University in North Carolina have made significant discoveries in the field of non-fossil fuel production.
Here is a summary of what has been achieved;
Search Begins for Missing Piece in Hydrogen Gas Enzyme
Chemists from the University of California, Davis and the University of Illinois have taken a big step towards recreating nature's most efficient tool for hydrogen gas generation.
Currently, synthesizing hydrogen for use as a fuel is an expensive and inefficient process, if researchers could find a way to copy the biological process of hydrogen production it would create a pathway towards replacing fossil fuels.

Nature builds and burns hydrogen with biological enzymes called hydrogenases. Original thinking was that there were active sites on each one, and that each site was made up of ten parts: four carbon monoxide molecules, two cyanide ions, two iron ions and two groups of a sulfur-containing amino acid called cysteine.
But by analysing the chemical composition of the enzyme, the team hypothesized that the composition was slightly differently. As the online journal Science Daily, explains, “The team discovered that it was instead more likely that the enzyme's engine was composed of two identical groups containing five chemicals: two carbon monoxide molecules, one cyanide ion, one iron ion and one cysteine group. The groups form one tightly bonded unit, and the two units combine to give the engine a total of 10 parts.”
However, when the team studied the lab-synthesized enzyme, they soon realized that something was wrong.
“Our recipe is incomplete,” says Thomas Rauchfuss, a professor of chemistry and the study’s co-author. “We now know that 11 bits are required to make the active site engine, not 10, and we are in the hunt for that one final bit.”
While the researchers have yet to work out which part is missing, they are continuing their work and hope that the discovery of an ‘assembly kit’ for the important iron-iron hydrogenase enzyme will inspire other chemists to search for the last ingredient.
Once this is known, catalyst design projects can begin to look into ways to industrially manufacture the enzyme to a level where it can begin to replace fossil fuels.
As Rauchfuss observes, “The take-away from this study is that it is one thing to envision using the real enzyme to produce hydrogen gas, but it is far more powerful to understand its makeup well enough to able to reproduce it for use in the lab.”
You can find out more about this breakthrough in the Proceedings of the National Academy of Sciences, where the team has published A Promising Mimic of Hydrogenase Activity.
Chemical Process Does in Lab What Trees Do in Nature
Another significant development was made recently, when a team of chemists from Wake Forest University in North Carolina were able to convert carbon dioxide into usable chemicals or fuels.
The process involved using silver diphosphide (AgP2) as a novel catalyst to convert carbon dioxide emitted from factories into syngas. While previous studies had already achieved the conversion of CO2 into a burnable fuel, this new process minimizes energy loss, making it more economically viable.
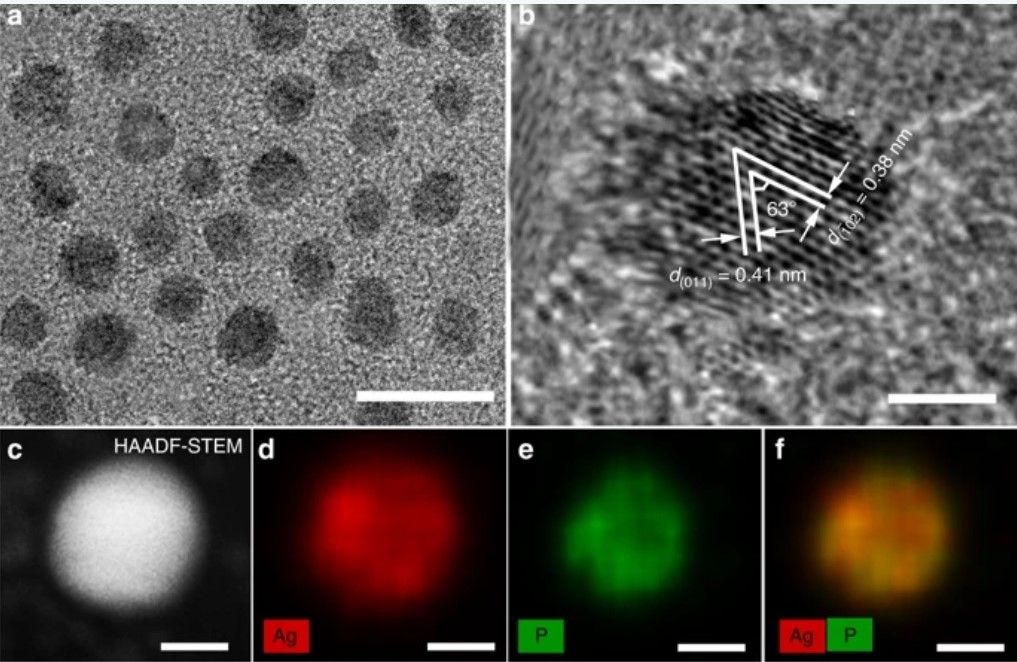
“This catalyst makes the process much more efficient,” explains Scott Geyer, author of the study ‘Colloidal Silver Diphosphide Nanocrystals as Low Overpotential Catalysts for CO2 Reduction to Tunable Syngas’. “Silver diphosphide is the key that makes all the other parts work. It reduces energy loss in the process by a factor of three.”
You can access the full study in the journal Nature Communications.
Ultimately, Geyer hopes to power the process with solar energy, resulting in an operation which converts sunlight into fuel, just like plants.

“People make syngas out of coal all the time,” says Geyer said. “But we're taking something you don't want, carbon dioxide pollution, and turning it into something you want, fuel for industry.”
Photo credit: ABC, Nature Communications, Arena, Renewablesnow, Intechopen, & H2view, stocksnap