The challenge for all producers of plastics, is making an affordable product that is sufficiently durable, yet also easily recyclable.
Biologically sourced plastic feedstock is frequently not strong enough for practical use in polymers, while fossil fuel-based raw materials can be too complex to breakdown and recycle.
The result is mountains of plastic waste that are too expensive to reuse, leading to burning or burying in landfill.

Researchers have now taken a step closer to solving this challenge by discovering a process to turn a natural molecule into different polymers which are easily recyclable into virgin monomer forms.
Tests have already shown that as much as 87% of the monomers can be recovered from the depolymerisation process, which chemists believe to be simple enough to allow for industrial up-scaling.
The study has now been published in the journal Matter, which highlights the discovery’s fundamental advantages.
· The new polymer can be chemically recycled in a closed loop.
· The depolymerization process can be conducted in a basic aqueous solution.
· The chemically recycled materials can be adjusted or have additives to reflect their new state.
The breakthrough was made via a cooperation between the Dutch University of Groningen and the East China University of Science and Technology (ECUST) in Shanghai, and is based on the natural molecule lipoic.
“We found a way to produce polymers from the natural molecule lipoic acid in a very controlled way,” explains Prof. Ben Feringa from the University of Groningen. “It is a beautiful molecule and a perfect building block that was created by nature.”
What is most significant about the molecule is that its central structure is shaped like a ring with a sulphur-sulphur bond. When this bond is broken, the sulphur atoms react with those of another monomer.
“This process was known before,” notes Feringa, “but we managed to find a way to control it and to create long polymers.”
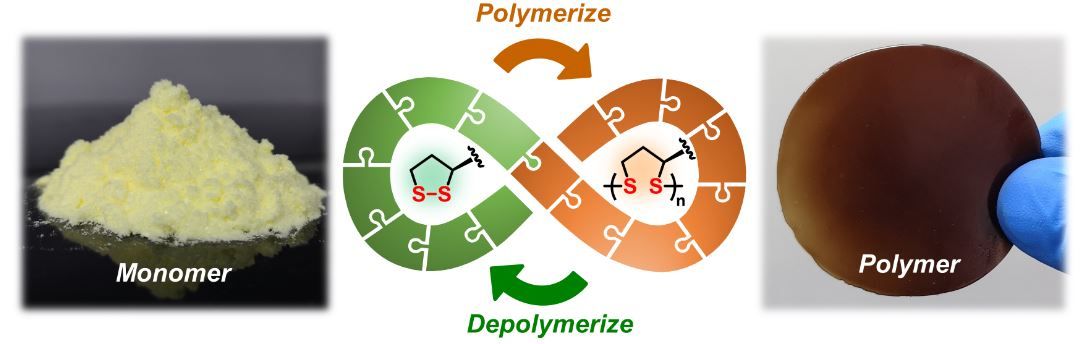
While this enables the creation of a versatile polymer, the real challenge is that the polymer bonds are also easily detachable and re-attachable – so the material can repair itself.
As the online journal Science Daily explains, “The molecule also has a carboxyl group, which readily reacts with metal ions. These can crosslink the polymers, which results in an elastic material. By dissolving the molecule in water with sodium hydroxide and then evaporating the water, a firmer polymer film is produced through ionic bonds. As the polymerization is achieved through reversible bonds, the material is also self-healing.”
“When it is cut,” says Feringa, “you can simply press the ends together and they will reconnect in a few minutes.”
It is a process that is fully reversible, turning the polymers back into their simpler form ready for reconnecting into a new plastic, without any loss of quality.
“Lipoic acid is a natural small molecule with an elegant structure,” describes postdoctoral researcher Qi Zhang who was at the forefront of the discovery. “We didn't have to do any tedious re-designing of the monomer to achieve a fully reversible polymerization.”
Instead, the polymers need only be exposed to sodium hydroxide to be dissolved into monomers.
“By adding a little acid, the monomers precipitate and can be recovered,” says Zhang. “The quality of these recycled monomers is identical to that of the original material.”

Work is now continuing to increase the percentage of monomers that are recoverable from the recycling process and also to look at how to advance the material so that it is suitable for practical use.
While this means that its application as a polymer raw material is still some way off, it is promising to find a new route towards sustainable plastics. A future industrial polymer which can easily and affordably be reused, even in different forms.
“We can recycle the material into monomers several times, without loss of quality,” concludes Feringa. “Our experiments show what is possible with these monomers.”
Photo credit: UniversityofGroningen, Nick Fewings on Unsplash, Marc Newberry, & Sigmund