Numerous research articles have focused on the possibility of using atmospheric or waste carbon dioxide as a feedstock for industrial chemical production.
The logic behind the research was perfect. Not only would chemical producers have a cheap and convenient source of raw material for making industrial chemicals, but mankind’s impact on climate change would be reduced as chemical manufacturers would either emit less CO2 or would even extract CO2 from the atmosphere.

Laboratory studies found that conversion was possible. Natural optimism combined with academic theory also supported the technology.
This led to the publication of articles such as ‘Researchers Just Found a Way to Turn CO2 Into Plastic With Unprecedented Efficiency’ and ‘Waste CO2 to be Turned into Ingredients for Fuel, Plastics and Even Food’ from popular scientific journals, such as Phys.org and Science Alert. Each outlining a not too distant future where carbon dioxide could be used to make numerous basic chemicals.
No longer would CO2 be a waste product or a pollutant in our atmosphere as the technology would exist to convert it into something useful.

However, the latest analysis of current technology has put grave doubts on the practicalities of large-scale conversion of carbon dioxide into a chemical feedstock.
The report has now been published in the journal ‘Proceedings of the National Academy of Sciences’, where the research team was able to, “… provide a global assessment of the technical climate change mitigation potential of carbon capture and utilization (CCU) in the chemical industry.” Namely, the team developed an engineering-level model of the global chemical industry and was used, “… to analyse the potential disruptive changes through large-scale CO2 utilization and resulting emission reductions.”
The study found that the technology could reduce greenhouse gas levels in the atmosphere by a massive 3.5 gigatonnes by 2030 (current global total emissions are estimated at 37.1 gigatonnes).

Unfortunately, to achieve this level of CO2 conversion requires an equally massive amount of energy.
As the journal New Scientist, states, “[The study found that] this would require 18.1 petawatt hours of electricity per year. That’s not much less than the 26 PWh of electricity a year produced by the entire world in 2018 according to the International Energy Agency. And only a third of global electricity currently comes from clean sources, including nuclear and hydroelectric power.”
Additionally noting that, “Even by optimistic growth projections, the total amount of renewable electricity available in 2030 is predicted to be less than 18 PWh.”
The problem, explains Andre Bardow of RWTH Aachen University in Germany who was a co-author of the study, lies in the laws of thermodynamics.
“What you are doing is inverting combustion,” says Bardow. This requires such a high level of energy that efforts would be best made in using the renewable electricity we have to reduce the emissions produced by even bigger polluters, namely heating and transport.
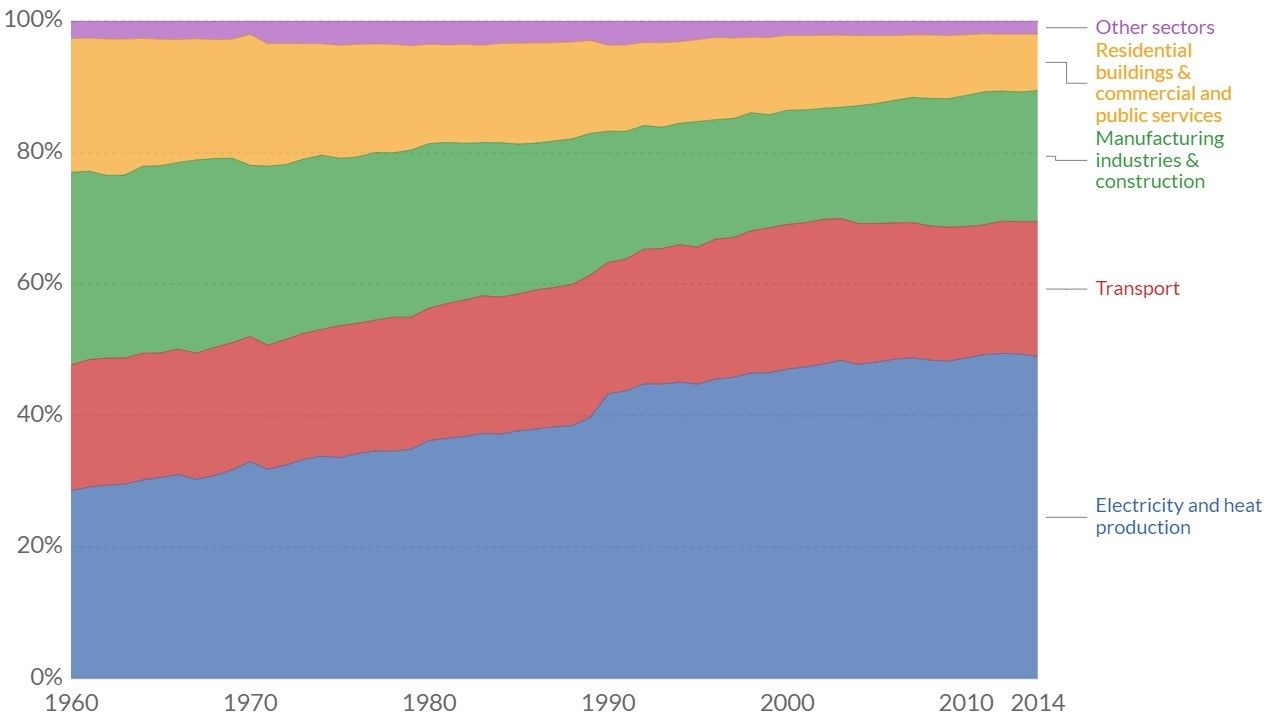
The study’s reasoning is logical, for while the chemical industry does produce 7% of global greenhouse gas emissions (20% of total industry output worldwide) spending so much energy to reduce the 7% to zero would not solve the challenges of climate change.
“A switch today would be a waste of valuable resources,” Bardow believes.
Instead, the chemical industry should focus on making other changes. Developing new technologies and focusing on energy use reduction may hold the key to slowdown global warming. Whereas unfortunately, when it comes to using carbon dioxide as a raw material for industrial chemical production the numbers simply don’t add up.
Photo credit: Ourworldindata, NBC, Henleybusinessschool, Enco2re, Edie, & Theconversation,