Polymer scientists have discovered a plastic that can be recycled, again and again and again and again, without any loss of quality or performance. Better still, the polymer can be broken-down at a molecular level and then reconstructed with new features, giving it new colour, shape, and/or texture.
One of the biggest problems with modern plastics is that most of it is never intended to be recycled. Only in the last few decades have polymer scientists begun looking into creating a more recyclable product. With the instigation of single-use plastic bans and the birth of the circular economy plastic producers have needed to put increasing value on the ability to reuse a polymer.

The challenge lies in the way that the monomers have additives, such as plasticizers, bound to them, making the process more complex and a little random.
When a plastic needs to be recycled, it is cut into tiny pieces and melted down ready to make a new product. But it is difficult to predict the qualities that the new material will retain from the original version. As the Berkeley Lab press release explains, “The inheritance of unknown and, therefore, unpredictable properties, has prevented plastic from becoming what many consider the Holy Grail of recycling: a 'circular' material.”
Currently, as a British Local Government Association (LGA) report from August 2018 states, “Only a third of plastic waste can be reused, with the rest being sent to landfill.”

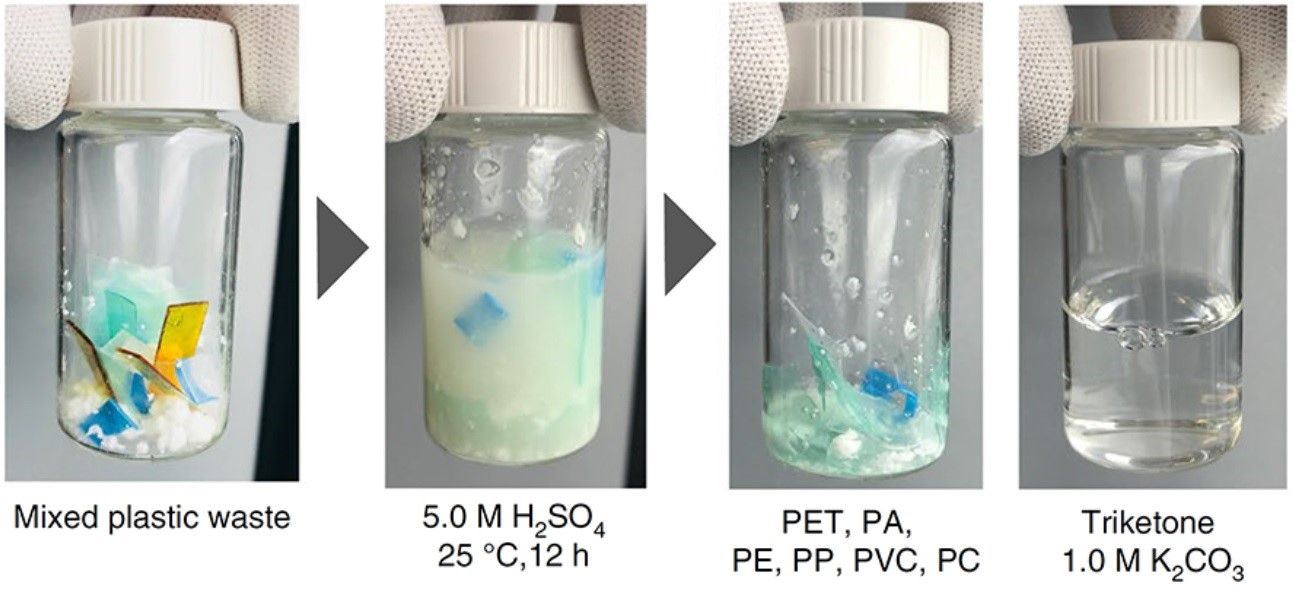
But now a team of researchers from the U.S. Department of Energy’s Lawrence Berkeley National Laboratory, who have solved the problem by developing the world’s first truly recyclable plastic. It is called poly(diketoenamine) or PDK.
The key, as lead author Peter Christensen, a postdoctoral researcher at Berkeley Lab’s Molecular Foundry explains, was in finding, “…a new way to assemble plastics that takes recycling into consideration from a molecular perspective.”
By discovering a polymer that contains reversible bonds, the researchers were able to recover the constituent monomers from the compounded additives by soaking the material in a very acidic solution.
As Norbert Sparrow, Editor in Chief at the industry journal Plastics Today, explains, “As is often the case, this discovery was somewhat accidental. Christensen was applying various acids to glassware used to make PDK adhesives when he noticed that the adhesive’s composition had changed. Upon examination of the sample’s molecular structure, Christensen discovered that it was composed of the original monomers. Further research demonstrated that, in addition to breaking down PDK polymers into monomers, the acid bath also separated the monomers from entwined additives.”

The team have now published their findings in the journal Nature Chemistry, where they state that, “Here, we show that next-generation plastics—polymerized using dynamic covalent diketoenamine bonds—allowing for the recovery of monomers from common additives, even in mixed waste streams. Poly(diketoenamine)s ‘click’ together from a wide variety of triketones and aromatic or aliphatic amines, yielding only water as a by-product.”
You can view a time lapse video of the degradation process here.
As an example of what can be achieved with PDK, the researchers presented an example of a computer keyboard that could be broken down into more elemental forms before being given additional properties, such as flexibility, and then upcycled into a watch strap.
The ability to completely reuse a plastic has come at a crucial time. As Brett Helms, a co-author of the study and a scientist at Berkeley Lab, makes clear, “We’re at a critical point where we need to think about the infrastructure needed to modernize recycling facilities for future waste sorting and processing. If these facilities were designed to recycle or upcycle PDK and related plastics, then we would be able to more effectively divert plastic from landfills and the oceans.”
Adding that, “This is an exciting time to start thinking about how to design both materials and recycling facilities to enable circular plastics.”

Beyond converting keyboards into watch straps, the team is now looking at specialised versions of PDK by adding a range of mechanical and thermal properties. They are also looking into how the polymer could be better designed to suit specific uses, such as in foam, 3D printing, or in the textile industry.
A further line of research is looking into how plant-based materials could be incorporated as a raw material, making the plastic an even greener product. Something that this whole project revolves around.
As the study’s authors state, “The ease with which poly(diketoenamine)s can be manufactured, used, recycled and re-used—without losing value—points to new directions in designing sustainable polymers with minimal environmental impact.”
Photo credit: Plasticstoday, Physicstoday, Greenmatters, BBC, & Phys.org